Acid Bath Murder - Exploring The Corrosive Truth
The phrase "acid bath murder" brings to mind something truly chilling, doesn't it? It conjures up a picture of something incredibly destructive, a process of breaking things down into their most basic forms. When we hear such a term, it's natural to wonder about the very substance at its core: the acid itself. What exactly is this chemical agent that gives such a grim phrase its power? We're going to take a look at what acids are, how they behave, and why they possess the capacity for such profound changes when they come into contact with various materials, like your typical metals or even living tissue.
So, the idea behind an "acid bath" isn't something pleasant to think about, yet it points directly to the fundamental properties of a certain kind of chemical. These substances, acids, have very distinct characteristics that make them capable of dissolving or altering many different things they touch. Understanding these characteristics helps us grasp the sheer potency that the phrase implies. It’s a bit like trying to understand a storm by studying the nature of wind and water, you know, rather than just seeing the damage it leaves behind.
In some respects, the concept draws our attention to the basic scientific definitions of acids. We're talking about chemicals that can taste quite sharp, like a lemon, or cause certain papers to change color. They also interact in specific ways with other elements, like metals, and with other chemical compounds known as bases. This exploration won't be about any specific event, but rather, about the fundamental chemistry that gives the very term "acid bath murder" its deeply unsettling resonance, and what makes these substances so reactive.
- Shannon Sharpe Standing Meme
- Aaron Tveit National Anthem
- Cade Cunningham Daughter Mom
- What Does Heaven Look Like
- King Von Coffin
Table of Contents
- What exactly is an acid?
- How do acids behave with other materials in an acid bath scenario?
- The Core Characteristics of Acids - What Makes Them So Potent?
- What happens when acids meet their opposites in an acid bath?
- Acids in Everyday Existence - A Wider View
- Are all acids the same strength in an acid bath context?
- The Chemistry Behind the Name - Acid Bath
What exactly is an acid?
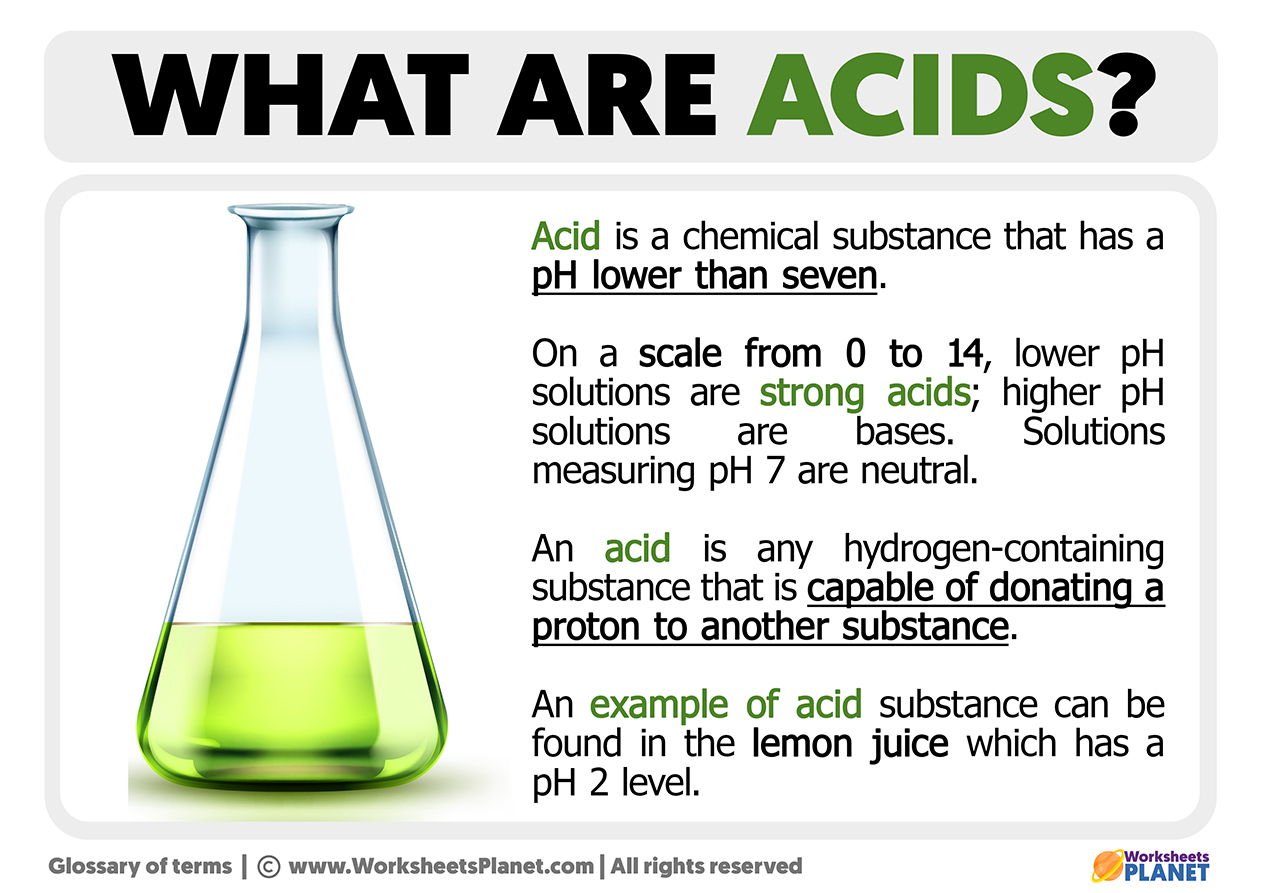
When you consider the fundamental nature of an acid, you're looking at a chemical substance that, when placed into a watery mixture, tends to have a sharp, tangy flavor. This is one of the most straightforward ways to describe it, as a matter of fact. Another simple way to tell if something is an acid involves a special kind of paper: if you dip a piece of blue litmus paper into an acidic liquid, it will quickly shift its hue to a bright red. This color alteration is a very common indicator, showing us the presence of these particular chemical agents. So, in basic terms, that's one part of the story.
From a more technical, scientific viewpoint, chemists have a couple of main ways to describe what an acid actually is. One popular definition suggests that an acid is a chemical entity that gives away hydrogen ions, which are also called protons. Think of it like a donor, passing along these tiny particles. Another way to look at it is that an acid is a chemical species that is ready to take in an electron pair. These definitions, you know, help scientists understand the deeper interactions happening at a molecular level, providing a more complete picture of how these substances operate.
Acids also have a very specific way of interacting with other types of chemical compounds, specifically those known as bases. When an acid and a base get together, they go through a process that creates new substances called salts. This interaction is often referred to as neutralization, because the acid's characteristic properties are, in a way, cancelled out by the base's properties. It's a pretty fundamental reaction in chemistry, and it helps us understand how acids can be managed or contained, which is important when thinking about something like an acid bath, for example.
- Que Jamon Es Bueno
- You Doing Great Sweetie
- Jon Bones Jones House Albuquerque
- Sadoer Marca De Donde Es
- Haeun And Yung Kai
How do acids behave with other materials in an acid bath scenario?
One of the striking behaviors of acids, particularly relevant to the chilling concept of an "acid bath murder," is their capacity to interact with certain types of metals. When an acid comes into contact with specific metallic elements, it can trigger a chemical reaction that results in the release of hydrogen gas. You might see bubbles forming as this gas escapes, which is, you know, a clear sign that a chemical transformation is taking place. This ability to break down metals is a key aspect of their potent nature, showing just how transformative these substances can be.
Furthermore, acids are well-known for their interactions with those chemical counterparts we mentioned earlier: bases. When an acid meets a base, they engage in a process called neutralization, and this interaction gives rise to what chemists call salts. This forming of salts is a pretty important chemical event, as it effectively balances out the distinct properties of both the acid and the base. In the context of an "acid bath," understanding this reaction is pretty crucial, as it explains how certain materials might be broken down or changed, and how the corrosive action could potentially be stopped or altered.
It's also worth noting that acids can appear in a wide variety of forms and possess quite different properties, even though they share the general characteristics of being acids. Some are incredibly strong and react very aggressively, while others are much milder. This variability means that their impact on different materials can differ quite a bit, depending on the specific acid being used. So, the kind of acid involved in something like an "acid bath" would determine, you know, the speed and intensity of its effects on whatever it came into contact with, which is a very significant detail.
The Core Characteristics of Acids - What Makes Them So Potent?
To truly grasp what makes acids so powerful, especially in a context like an "acid bath," we can look at their fundamental traits. As we've touched upon, they are recognized for having a sharp, tangy flavor, much like the zest of a lemon. They also possess that notable quality of making certain indicator papers, like litmus, shift their color to a deep red. Moreover, they are known for their ability to react with various metallic substances, and with carbonate compounds too, often causing a fizzing or bubbling as they break things down. These are, you know, the signature signs that we are dealing with an acid, showing their inherent reactivity.
Interestingly, the term "acid" also appears in an entirely different field: computer science. In that specific context, "ACID" stands for atomicity, consistency, isolation, and durability, which are a collection of characteristics for database transactions meant to ensure the accuracy of data even if there are mistakes or power failures. However, it's pretty important to be clear that this computer science meaning has absolutely nothing to do with the chemical substances we're discussing here. We're talking about the chemical acid, the one that can be sour and reactive, not the set of rules for computer data, you know, which is a completely separate concept.
In simple, everyday terms, acids are chemical compounds that taste quite sharp or tart, and they have the capacity to turn blue litmus paper a distinct red color, which is a clear signal of their acidic nature. They are also known for their ability to engage in chemical interactions with bases, creating new compounds in the process. Basically, when you hear about acids, you should think of these core properties: a particular taste, a specific color change on indicator paper, and a readiness to react with other materials, which, you know, defines their chemical identity.
What happens when acids meet their opposites in an acid bath?
When acids encounter bases, their chemical opposites, a very specific and important interaction takes place, which is something to think about in any discussion involving an "acid bath." This interaction leads to the creation of what are known as salts. It's a bit like two different pieces fitting together to make something new. This formation of salts is a direct result of the acid's ability to give away those hydrogen ions and the base's capacity to accept them, or vice versa, depending on the specific chemical definitions you're using. So, in essence, they combine to form a different kind of substance, which is pretty interesting.
This process is generally referred to as neutralization. During neutralization, the distinct, often corrosive, properties of the acid are, more or less, cancelled out by the properties of the base. The resulting salt often has a neutral pH, meaning it's neither acidic nor basic. This chemical balancing act is a key concept in chemistry and, you know, it explains why certain substances can be used to counteract the effects of an acid. It's how we might clean up an acid spill, for instance, by adding a basic substance to make it less harmful, which is quite practical.
The outcome of this reaction – the formation of a salt – is a fundamental aspect of acid-base chemistry. It shows how these two types of compounds are intrinsically linked and how their interaction leads to a different chemical state. This principle is, frankly, at the heart of many chemical processes, from industrial applications to what happens inside our own bodies. So, when thinking about the dramatic effects implied by an "acid bath," understanding this neutralization process gives us a clearer picture of the chemical dynamics at play, and how things could be broken down or transformed.
Acids in Everyday Existence - A Wider View
While the term "acid bath murder" points to a very extreme and dark application, acids are, in fact, a very common part of our daily lives, usually in much milder forms. Think about the refreshing tang of a lemon or the sharp taste of vinegar; these are both examples of common acids we consume or use around the house. Citric acid gives citrus fruits their characteristic flavor, and acetic acid is what makes vinegar, you know, so distinct. These everyday examples help illustrate that acids aren't always dangerous or destructive, but rather, they possess a range of properties depending on their type and concentration.
Acids also play a pretty significant role in various industrial processes. They are used in cleaning products because of their ability to dissolve certain materials, helping to remove grime and rust. In manufacturing, acids are essential for producing everything from fertilizers to plastics. For instance, sulfuric acid is a highly important industrial chemical, used in making car batteries and in many chemical synthesis processes. So, these substances are truly fundamental building blocks in modern industry, showing their widespread utility beyond any grim association, which is quite remarkable.
Even our own bodies rely on acids for essential functions. Our stomachs, for example, produce hydrochloric acid, which is incredibly important for breaking down the food we eat and helping with digestion. This stomach acid is strong enough to dissolve food, but our bodies have mechanisms to protect themselves from its corrosive effects. This natural presence of acids within us, you know, highlights their biological importance and shows that, in the right context and concentration, they are absolutely vital for life, not just for their more dramatic and destructive capabilities.
Are all acids the same strength in an acid bath context?
It's a common misconception that all acids are equally potent, especially when you consider the dramatic implications of a phrase like "acid bath murder." In reality, acids come in a spectrum of strengths, typically categorized as either strong or weak. A strong acid, like hydrochloric acid or sulfuric acid, gives away nearly all its hydrogen ions when put in water, making it incredibly reactive and corrosive. A weak acid, on the other hand, like acetic acid found in vinegar, only gives away a small fraction of its hydrogen ions, meaning it's far less aggressive. This difference in strength is, you know, a very important factor.
The concentration of an acid also plays a huge part in its overall effect. Even a relatively weak acid can become quite dangerous if it's highly concentrated, meaning there's a large amount of the acid dissolved in a small amount of water. Conversely, a strong acid that is very diluted, with a lot of water added, might be less harmful than a concentrated weak acid. So, when considering the impact of an acid in a scenario like an "acid bath," both the inherent strength of the acid and its concentration are absolutely critical factors that determine its destructive potential, which is pretty complex.
These differences in strength and concentration significantly impact how acids react with various materials. A strong, concentrated acid will break down organic matter and metals much more quickly and completely than a weak or diluted one. This means that the specific type and preparation of the acid would dictate the speed and effectiveness of any "acid bath" process. Understanding this variability is key to appreciating the nuances of acid chemistry, and it helps explain why some acids are used for gentle cleaning while others are, you know, considered extremely hazardous and capable of profound chemical transformation.
The Chemistry Behind the Name - Acid Bath
The very name "acid bath" speaks to the inherent chemical properties of acids, particularly their capacity for dissolving and reacting with other substances. When we consider the definitions we've discussed – acids tasting sour, changing litmus paper, reacting with metals to free hydrogen, and combining with bases to form salts – we begin to understand the basis for such a grim concept. It's the ability of these substances to break down materials, sometimes very rapidly and completely, that gives the term its powerful and unsettling imagery. So, the name itself is, you know, deeply rooted in the actual chemical behavior of acids.
The sheer destructive capacity inherent in certain types of acids is truly remarkable. Some acids, especially strong and concentrated ones, can break down organic compounds, including living tissue, and corrode many metals. This destructive power stems from their chemical structure and their readiness to engage in reactions that essentially pull apart the molecular bonds of other materials. This is why, for instance, certain acids are used in laboratories to dissolve samples or in industrial settings for very powerful cleaning. It’s a testament to their chemical aggression, which is quite potent.
Ultimately, the term "acid bath" exists to describe a particularly grim and effective method of material destruction, chosen because of the well-known, potent chemical properties of acids. It highlights the corrosive nature of these substances and their ability to reduce things to their basic components. The phrase, therefore, serves as a stark reminder of how fundamental chemical properties can be applied in ways that are deeply unsettling, illustrating the extreme end of what acids are capable of doing when used with harmful intent. It’s, you know, a very stark example of chemistry in action.
- Tribal Braids With Sew In The Back
- Shrimpy The Bulldog
- Hannah Montana Purple Outfit
- Fenix Flexin Mike Sherm
- Maury Memes You Are Not The Father

What is an Acid in Chemistry? - The Chemistry Blog
Acids Chemistry

Fabric Dye Chemistry Experiment Recently updated ! - TrendRadars